Upstream Bioprocessing
Biological products (biologics), such as therapeutic proteins, vaccines, or cell and gene therapy products represent a new medical paradigm. Those therapies can provide remarkable outcomes for patients and have already revolutionized the treatment of many diseases in a variety of fields.
Biologics manufacturing is challenging
Therapeutic manufacturing processes can be separated into upstream processes and downstream processes. The upstream process is defined as the entire process from early cell isolation and cultivation, to cell banking and culture expansion of the cells until final harvest – in other words the termination of the culture and collection of the live cell batch for purification.
Because the production is reliant on living, extremely sensitive cells for production, biologics are much harder to manufacture than small-molecule drugs. Their immunogenicity, adverse events, and efficacy can all be affected by even the slightest manufacturing process change. As such, it is very difficult to scale up from research to clinical quantities and many promising therapies can not reach the market. In addition, the most significant drawback is low product yield, followed by the costs related to the extensive cell-line development and limited cell viability for production. In all, the manufacturing process is extremely expensive and biologics are not accessible to all patients.
Introducing the Cellevat3d™ platform
Cellevate´s core nanotechnology is single use, sustainable, scalable and innovative nanofiber-based solution for cell culture. This is a game-changing deep-tech innovation for the biopharmaceutical industry. The Cellevat3d™ platform is highly customizable, faithfully replicates the 3D extracellular environment found in the human body, and provides an exceptional surface area for cell growth compared to competing solutions on the market.
Three reasons to choose Cellevat3d™
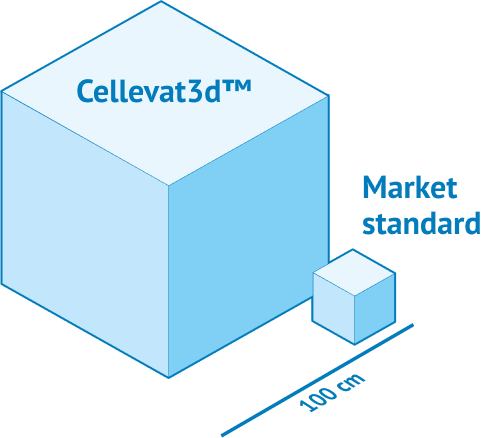
More surface
Exceptional surface-to-volume ratio.
The platform offers an incredibly high specific surface area (>5 m2/g) that increases the total biomass that can be maintained in your system, enhancing production of cellular products.
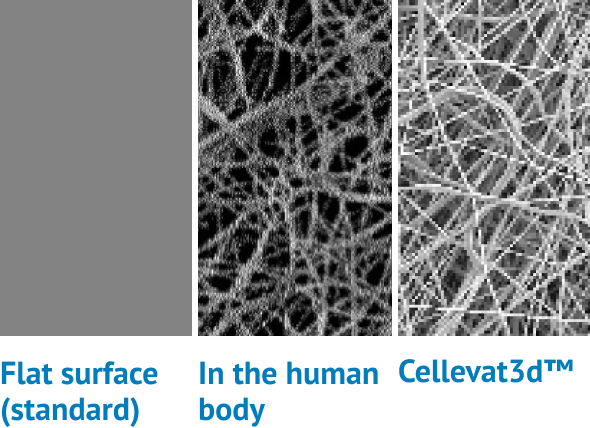
Better environment
Cellevate’s nanofibers effectively mimic the collagen and elastin fibers that make up the human extracellular environment. As a result, cells are allowed to behave naturally: proliferating, migrating, and interacting with one another in 3D.
Full scalability
Cellevate’s production method allows both free-form and volume defined production. Thus, the adaptability of the macro format of the nanofiber material, and the customizable fiber properties, creates a complete system and a platform for a whole range of revolutionary products for production of biologics and for cell-based therapies.

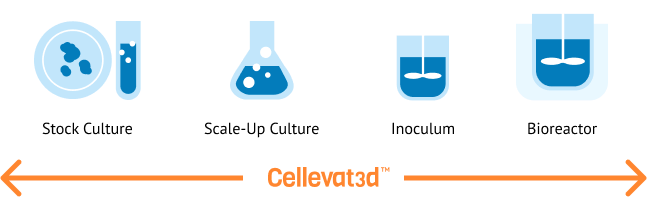
Cellevate is currently using the Cellevat3d™ platform to develop a new product portfolio, Cellevat3d™ Microcarriers – supportive structures for cell growth and expansion – as the nanofiber-based materials are perfectly suited for upstream processes in cell- and gene therapy applications. The product portfolio covers all cell culture process steps.
Successful initial validation in industrial applications
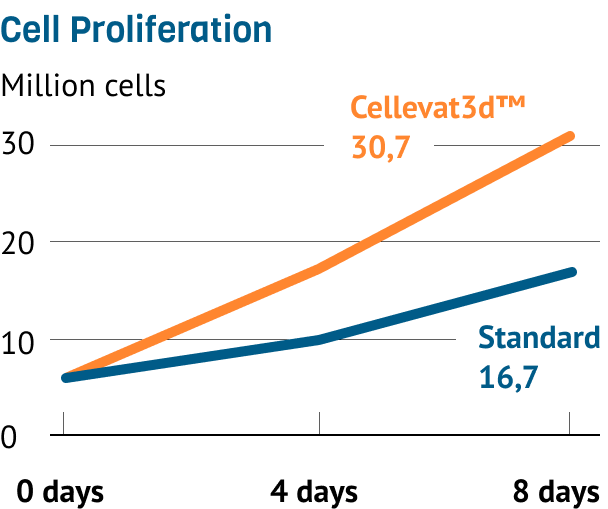
In a study spanning 8 days, HEK293 AD cells have been shown to proliferate faster on Cellevat3d™ materials than commercially available standard.
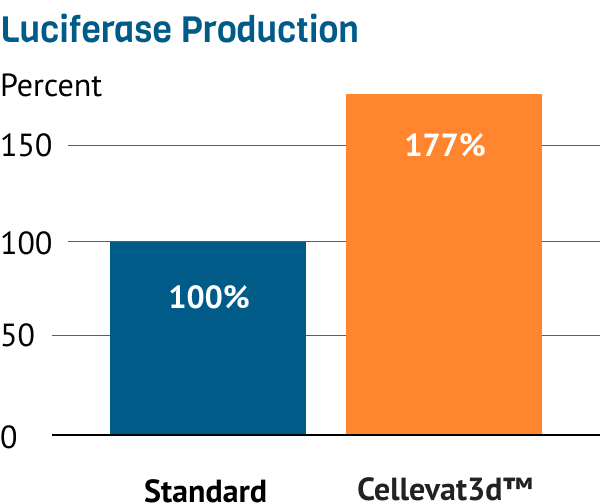
Additionally, HEK293 AD on Cellevat3d™ materials produce ~2x more reference protein than on commercially available standard after 8 days.
By offering high yields and ensuring the optimal conditions for cell growth, the Cellevat3d™ platform have the potential to transform the biopharmaceutical market by pushing the limits of today’s biological drugs production.


